Trigonal Pyramidal Geometry: Unveiling Molecular Shapes
Ever wondered how the shapes of molecules dictate their behavior, influencing everything from the medicines we take to the materials that shape our world? The key lies in understanding the intricacies of molecular geometry, and one of the most fundamental shapes is the trigonal pyramidal geometry.
In the captivating world of chemistry, where atoms dance to the tune of electrons and bonds, the three-dimensional arrangement of atoms within a molecule is not merely a matter of aesthetics; it's a determinant of function. This arrangement, or molecular geometry, dictates how a molecule interacts with others, influencing its physical and chemical properties. Among the myriad of shapes molecules can take, trigonal pyramidal geometry stands out for its prevalence and its impact on a vast array of compounds, including ammonia, a cornerstone of the fertilizer industry and a vital component in countless chemical processes. This geometry arises when a central atom bonds with three other atoms and possesses one lone pair of electrons. Understanding it is not just an academic exercise; it is crucial for any in-depth understanding of chemistry.
| Category | Details |
|---|---|
| Definition | The three-dimensional arrangement of atoms in a molecule where a central atom bonds with three surrounding atoms and also possesses one lone pair of electrons. |
| Central Atom Characteristics | Forms three covalent bonds and has one lone pair of electrons. |
| Bonding Atoms | Typically, three atoms surround the central atom. |
| Lone Pair | One lone pair of electrons resides on the central atom. |
| Shape | Resembles a pyramid with a triangular base. |
| VSEPR Theory Influence | The lone pair repels bonding pairs, affecting bond angles. |
| Bond Angle | Typically around 107 (e.g., in ammonia), less than the ideal tetrahedral angle of 109.5. |
| Examples | Ammonia (NH), Phosphine (PH), Chlorate Ion (ClO). |
| Polarity | Most trigonal pyramidal molecules are polar. |
| Reactivity | Lone pairs can participate in chemical reactions, increasing reactivity. |
| Applications | Ammonia production, drug design, catalysis. |
| Significance | Important in explaining molecular behavior, predicting interactions, and biological processes such as enzyme catalysis. |
Reference: https://chem.libretexts.org/Bookshelves/General_Chemistry/Map%3A_General_Chemistry_(Petrucci_et_al.)/09%3A_Molecular_Geometry/9.02%3A_Molecular_Geometry
- Unveiling Patrick The Stripper Who Redefined Performance Art
- Llama Llama Red Pajama The Timeless Charm Impact
When a central atom is bonded to three other atoms and possesses a lone pair of electrons, the resulting geometry is trigonal pyramidal. This shape arises from the principles of Valence Shell Electron Pair Repulsion (VSEPR) theory, which dictates that electron pairs, both bonding and non-bonding, will arrange themselves around the central atom to minimize repulsion. The lone pair, with its higher electron density, exerts a greater repulsive force than the bonding pairs, compressing the bond angles slightly and distorting the ideal tetrahedral shape.
Understanding trigonal pyramidal geometry is vital for several reasons. First, it provides a framework for predicting and explaining the physical and chemical properties of molecules. For example, the presence of a lone pair in this geometry often leads to molecular polarity, which in turn affects solubility, reactivity, and intermolecular forces. Second, it plays a pivotal role in understanding chemical reactions. The lone pair can participate in reactions, acting as a site for nucleophilic attack or serving as a source of electron density. Lastly, it is fundamental in understanding the behavior of molecules in various environments, including biological systems, where this geometry is prevalent in crucial molecules like amino acids and enzymes.
The exploration of trigonal pyramidal geometry begins with understanding its fundamental characteristics. The defining features include a central atom that forms three covalent bonds with surrounding atoms and harbors one lone pair of electrons. This lone pair is the key to understanding why the molecule takes on a pyramidal shape rather than a flat, trigonal planar shape. The three bonded atoms form a triangular base, and the lone pair sits at the vertex, creating a structure that, in essence, is a pyramid.
- Starlight Boys Final Lineup A Deep Dive Into Their Legacy
- Tiktok Wallpapers Trends Tips Designs For Your Phone
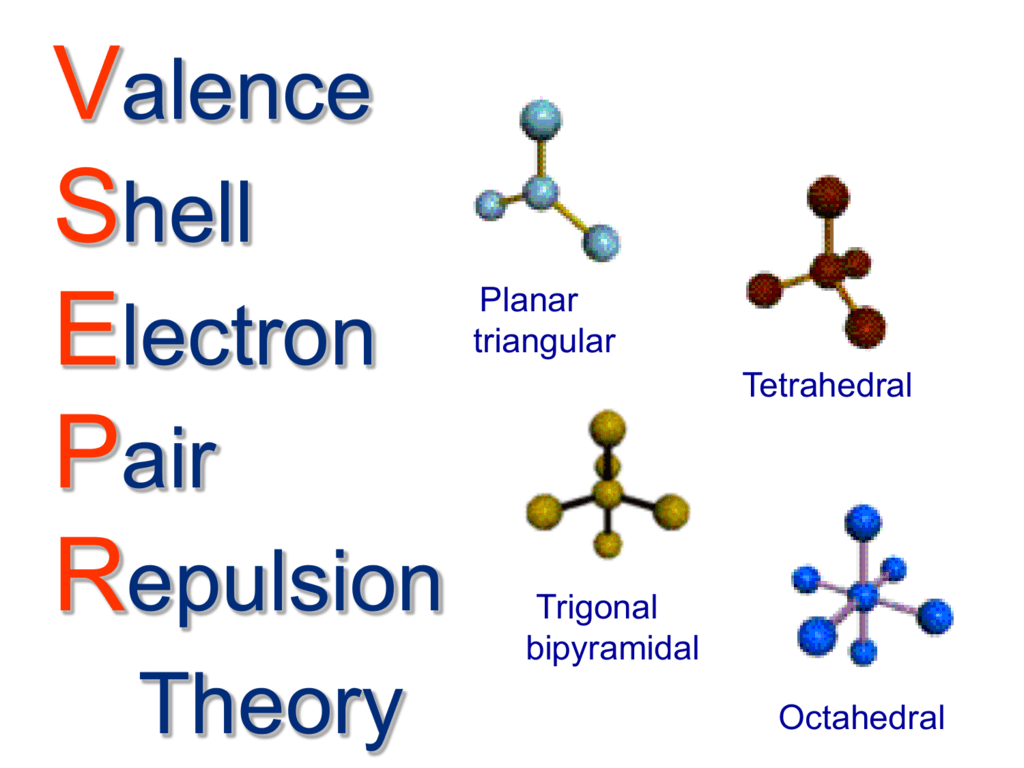
The Valence Shell Electron Pair Repulsion (VSEPR) theory is the cornerstone for understanding the trigonal pyramidal shape. VSEPR theory states that electron pairs, whether involved in bonding or existing as lone pairs, repel each other and arrange themselves to minimize this repulsion. In trigonal pyramidal molecules, the lone pair of electrons exerts a stronger repulsion than the bonding pairs due to its greater electron density, pushing the bonding pairs closer together. This repulsion is what causes the bond angles to deviate from the ideal tetrahedral angle of 109.5 degrees. In molecules like ammonia (NH), the bond angle is approximately 107 degrees.
Several well-known molecules exhibit trigonal pyramidal geometry, each showcasing different nuances of this molecular shape. Ammonia (NH), with its nitrogen atom bonded to three hydrogen atoms and a lone pair of electrons, is a textbook example. The presence of the lone pair influences the bond angles, pushing the hydrogen atoms closer together, resulting in the bond angle of approximately 107 degrees. This geometry makes ammonia polar, which is crucial for its role in various chemical processes.
Phosphine (PH), another example, has a phosphorus atom as the central atom bonded to three hydrogen atoms and a lone pair of electrons. Although the overall structure is similar to ammonia, the bond angles are slightly smaller due to the larger size of the phosphorus atom compared to the nitrogen atom. This difference highlights how the size of the central atom affects the molecular geometry and, consequently, the molecule's properties. The chlorate ion (ClO), where a chlorine atom is bonded to three oxygen atoms and has one lone pair, provides yet another instance. The bond angle here is around 101 degrees, showcasing that the type of atoms involved also plays a role.
The bond angle in trigonal pyramidal molecules is not a fixed value but is influenced by various factors. The size of the central atom, the electronegativity of the surrounding atoms, and the number and type of electron pairs all play a role. As mentioned, the presence of a lone pair decreases the bond angle because of the greater repulsion between the lone pair and the bonding pairs. For instance, the bond angle in ammonia (NH) is approximately 107 degrees, while in phosphine (PH), it is slightly smaller. The electronegativity of the surrounding atoms also influences the bond angle. More electronegative atoms pull the bonding electrons closer to themselves, thus reducing the repulsion between the bonding pairs and leading to slightly larger bond angles.
Trigonal pyramidal molecules have several distinctive properties that are directly linked to their geometry. Most importantly, the presence of a lone pair of electrons on the central atom leads to polarity. This uneven distribution of charge gives these molecules the ability to interact with other polar molecules, influencing their solubility in polar solvents like water. In addition, the lone pair can participate in chemical reactions, making these molecules more reactive than their counterparts with different geometries. This reactivity is critical in several chemical and biological processes, from industrial applications to biological systems.
To fully appreciate trigonal pyramidal geometry, it is useful to contrast it with other molecular geometries. In tetrahedral geometry, which occurs when a central atom is bonded to four surrounding atoms and has no lone pairs, the bond angle is 109.5 degrees. The absence of lone pairs in tetrahedral molecules means there is no extra repulsion to compress the bond angles. On the other hand, trigonal planar geometry occurs when a central atom is bonded to three surrounding atoms and has no lone pairs. In this case, the bond angle is 120 degrees. Understanding these differences is crucial for predicting a molecule's behavior and reactivity.
The applications of trigonal pyramidal geometry are vast and span several disciplines. Ammonia production is a significant industrial application. The unique properties of ammonia, stemming from its trigonal pyramidal shape, make it an essential component in fertilizers and industrial chemicals. In drug design, many pharmaceuticals incorporate trigonal pyramidal molecules because their shape and polarity allow them to interact effectively with biological targets. Furthermore, these molecules can serve as catalysts in chemical reactions, enhancing the efficiency of reactions because of their reactivity and polarity.
The importance of trigonal pyramidal geometry is immense in both chemistry and biology. In chemistry, it helps to explain how molecules behave in different environments and predict how they will interact. In biology, it plays a critical role in countless processes, including enzyme activity and protein folding. Enzymes often feature trigonal pyramidal active sites that bind to specific substrates, enabling catalytic activity. The shape and the presence of a lone pair are essential for the function of these active sites. Moreover, the geometry of amino acids and nucleotides can influence the structure of proteins and DNA, therefore affecting biological processes.
- Dtis Favorite Items Boost Philippine Economy Empower You
- Emma Christina Newell From Dreams To Global Icon
Trigonal Pyramidal
Trigonal Pyramidal Structure
Trigonal Pyramidal Lewis Structure